Valence Electrons Brf5
By Post
In the case of H2O, the O has larger electronegativity. We started with 23 electrons but in the diagram we've 24. We’ve added an electron to complete it for the octet rule and due to that additional electron, that's the reason it is -1. Before we proceed speaking about bonding, we have to talk about what urges atoms to do it. And since oxygen likes the attention, we’ll use it as a reference for this whole lesson.
The inner transition metals are the weather within the two rows which may be most often shown beneath the the rest of the other components within the periodic desk. It is safest to keep in mind that the inner transition metals have three valence electrons, however as is usually the case, there are a few exceptions to that rule. For brevity, many chemists report the electron configuration of an atom by giving only its outermost subshell, like 4 s 1 for potassium or 4 s 2 for calcium.
So for the sulfate ion - SO42- - two additional valence electrons have to be added to the valence electron count. And for polyatomic cations , electrons must be subtracted from the valence electron depend. So for the ammonium ion - NH4 - one valence electron have to be subtracted from the valence electron rely.
What Are The Valence Electrons Of Iron?
D- block parts have 1-10 electrons in the d- shell. But the (n 1)th S outer shell has either 2 or 1 electron in order to finish half filled or full stuffed states of the weather which have one electron lower than completion of that state. Similarly, Group 3 - Group eight are where the $p$ orbital is being filled up. All the Group 5 atoms thus all have an outermost $p$ orbital full of three electrons.
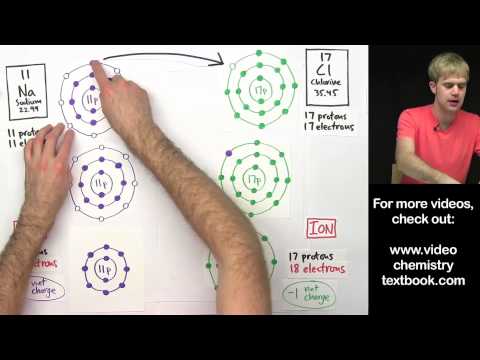
This effective charge is also felt by any valence electrons from different atoms, which is the main cause why secure bonds can occur. This instructor led activity will produce a partially crammed periodic desk that accommodates electron-dot fashions for the first twenty components in the acceptable boxes valence electrons in chromium. It might be used as a visible tool for college students to connect ideas similar to valence electrons and group properties, atomic radius tendencies, ionic and covalent bonding areas and others. Generally, the valence electrons are the electrons in the outermost shell — in different phrases, the last electrons added.
For example, if a component is positioned in Group 2, it might have 2 valence electrons. Elements which are positioned in Group 17, would have 7 valence electrons. Hence, the transition parts (i.e d-block components from group three to 12) can have more valence electrons ranging from 3 to 12. In this case, the valence electrons of copper are two. But this electron configuration in copper just isn't correct.
First Known Use Of Valence Electron
On the other hand, hydrogen acquires the electron configuration of helium and comes to a steady state. In this way, hydrogen and oxygen make water via electron sharing. The valence electrons of all the weather on this group, together with magnesium are two. These round pathways are referred to as shell or energy levels. According to scientist Niels Bohr, power levels are three-dimensional spherical. That is, the shells are considerably like football-shaped spheres.
- Generally, on a periodic desk, all the elements in a single vertical column will have the same number of valence electrons.
- The parts that type bonds by donating electrons are referred to as cation.
- We began with 23 electrons however in the diagram we've 24.
- The number of shells round an element’s nucleus is indicated by the period quantity .
The desk below represents the variety of valence electrons current within the completely different teams of the Periodic Table. Therefore, the easiest method to decide such electrons is by checking out the elements’ place in the periodic table. When the magnitude of the electronegativities of the primary group components is added to the periodic table as a 3rd axis, we get the results proven in the figure under. Halogenation is a course of during which halogens which are Cl, Br, I, F are introduced in a molecule. In the halogenation of alkenes the halogen atoms are added into π bond system. A s the pi bond is the region of excessive charge density so it act as nucleophilic middle.
What Number Of Valence Electrons Does Chlorine Cl Have?
Another way to find or determine these electrons is thru the atomic number. When the difference between the electronegativities of the elements in a compound is relatively giant, the compound is best categorized as ionic. By overexposing to phosphorus, workers in match factories had been developed a painful and debilitating deformation of jawbone often recognized as phossy jaw. Phosphorus compounds are additionally utilized in chemical industry for applications in delicate https://www.sciencedaily.com/releases/2019/09/190909131144.htm drinks, flame retardants and prescription drugs . The maximum number of univalent atoms that may combine with an atom of the factor into consideration, or with a fraction, or for which an atom of this element may be substituted. The table below reveals the Group characteristics for the first 20 components (Hydrogen-atomic number 1 to Calcium-atomic quantity 20) of the Periodic Table.
